Dye-sensitized hydrogen evolution using a semiconductor combined with a redox photosensitizer has long been studied as a potential means of solar-to-fuel conversion, since the basic principle of spectral sensitization, or the expansion of the sensitivity of a semiconductor to the visible spectra was introduced.78 When the sensitization of a large band gap semiconductor to the visible region is achieved with a dye at the molecular level, it is termed dye-sensitization. Dye-sensitized TiO2 has many photocatalytic applications including hydrogen generation under visible light in the presence of a sacrificial reagent, and the degradation of a wide range of compounds, such as aliphatic and aromatic compounds in aqueous mediums.
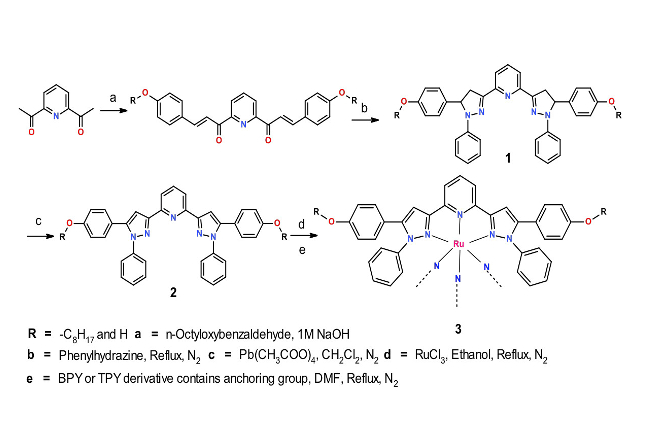
Research Approach. We plan to utilize the ruthenium(II) complex of bis(3-(2,6-pyridyl)-2-pyrazolyl derivative as a photosensitizer. The possible anchoring groups are 2,2-bipyridine-4,4-dicarboxylicacid and 2,2-bipyridine-4,4-diphosphoricacid. The ligand bis(3-(2,6-pyridyl)-2-pyrazolyl derivative is synthesized by a slight modification in the earlier literature.Long alkyl chains adorn this new pyrazolyl ligand to limit solubility and promote stability of the ruthenium dye complexes in dye-sensitized solar cell applications.
Dipyrazolylpyridine based pyrazoline as a fluorescent probe. Highly fluorescent dihydro-derivative of pyrazole (pyrazoline) is another important aza-heterocycle that has been extensively researched. The ring is quite stable and has inspired chemists to carry out various structural variations in the ring. This has propelled the development of diverse pyrazolines which will act as solvatochromic probes, electroluminescent compounds and chemosensors. The R-group in pyrazoline derivative can be modified based on our needs. This compound is a promising scaffold to develop a ratio-metric sensor to detect toxic metal ions in aqueous medium.