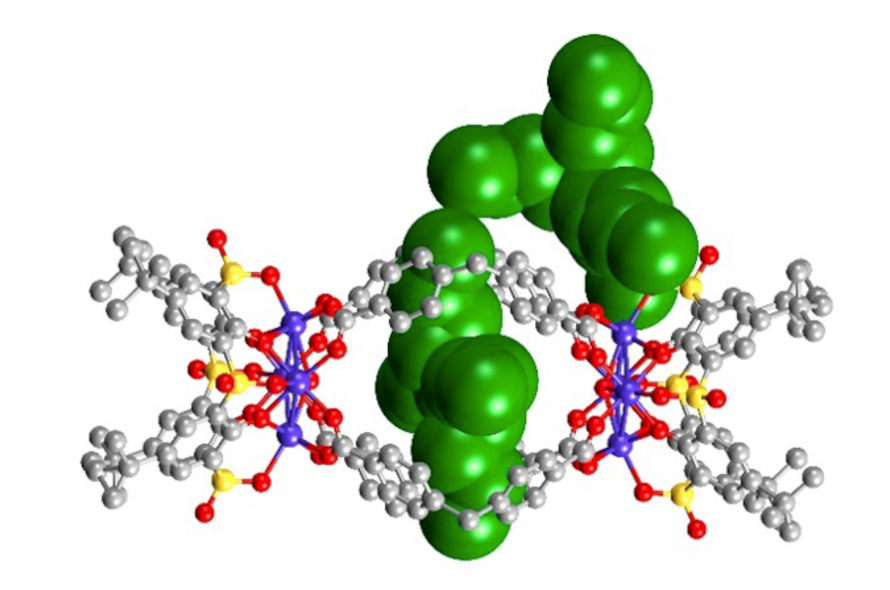
Research in the Wang laboratory focuses on investigating the chemistry of a new class of nanostructured molecules termed metal-organic supercontainers (MOSCs), which can be constructed from various metal ions such as Co2+, Ni2+, Zn2+, etc., organic linkers (e.g., 1,4-benzenedicarboxylate), and cup-shaped container precursors based on sulfonylcalix[4]arenes. The most attractive features of the MOSCs are exemplified by their tunable structure, multiple-cavity architecture, and enzyme-like binding pocket. These important characteristics allow the MOSCs to trap, transport, and transform (“T3”) an array of small molecules, such as CO2, organic compounds, pharmaceuticals, and neurotransmitters. The ability of MOSCs to encapsulate and manipulate these small-molecule guests is reminiscent of the receptor-ligand binding in biology and has led to exciting applications in several areas, including carbon capture, supramolecular catalysis, drug delivery, and therapeutics for substance addiction.
Selected Publications
1. Dai, F.-R.; Wang, Z., Modular Assembly of Metal–Organic Supercontainers Incorporating Sulfonylcalixarenes, J. Am. Chem. Soc. 2012, 134, 8002-8005; DOI:10.1021/ja300095j
2. Dai, F.-R.; Sambasivam, U.; Hammerstrom, A. J.; Wang, Z., Synthetic Supercontainers Exhibit Distinct Solution versus Solid State Guest-Binding Behavior, J. Am. Chem. Soc. 2014, 136, 7480-7491; DOI:10.1021/ja502839b
3. Qiao, Y.; Zhang, L.; Li, J.; Lin, W.; Wang, Z., Switching on Supramolecular Catalysis via Cavity Mediation and Electrostatic Regulation, Angew. Chem. Int. Ed. 2016, 55, 12778-12782; DOI:10.1002/anie.201606847
4. He, C.; Chen, X.; Sun, C.-Z.; Zhang, L.-Y.; Xu, W.; Zhang, S.; Wang, Z.; Dai, F.-R., Decahexanuclear Zinc(II) Coordination Container Featuring a Flexible Tetracarboxylate Ligand: A Self-Assembly Supermolecule for Highly Efficient Drug Delivery of Anti-Inflammatory Agents, ACS Appl. Mater. Interfaces 2021, 13, 33812-33820; DOI:10.1021/acsami.1c06311